Hi-Tech Products Die Cutting and Converting
Hi-Tech Products Die Cutting and Converting
Call: (714) 670-2150 ISO 13485:2016 Certified

Lateral Flow Backing Cards
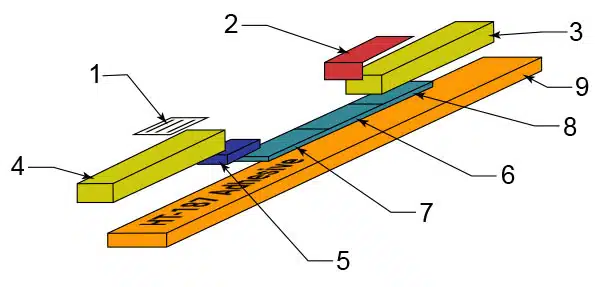
Hi-tech products manufactures HT-187, a high quality, competitively-priced acrylic pressure sensitive adhesive for IVD backing cards. Specifically engineered for in vitro test strips and lateral flow devices. This custom formulated adhesive is compatible with all types of membranes, wicking material, conjugated pads and filter media. HT-187 is identical to GL-187. HT-187 is widely used in both the US and International markets for lateral flow backing card manufacturing.

